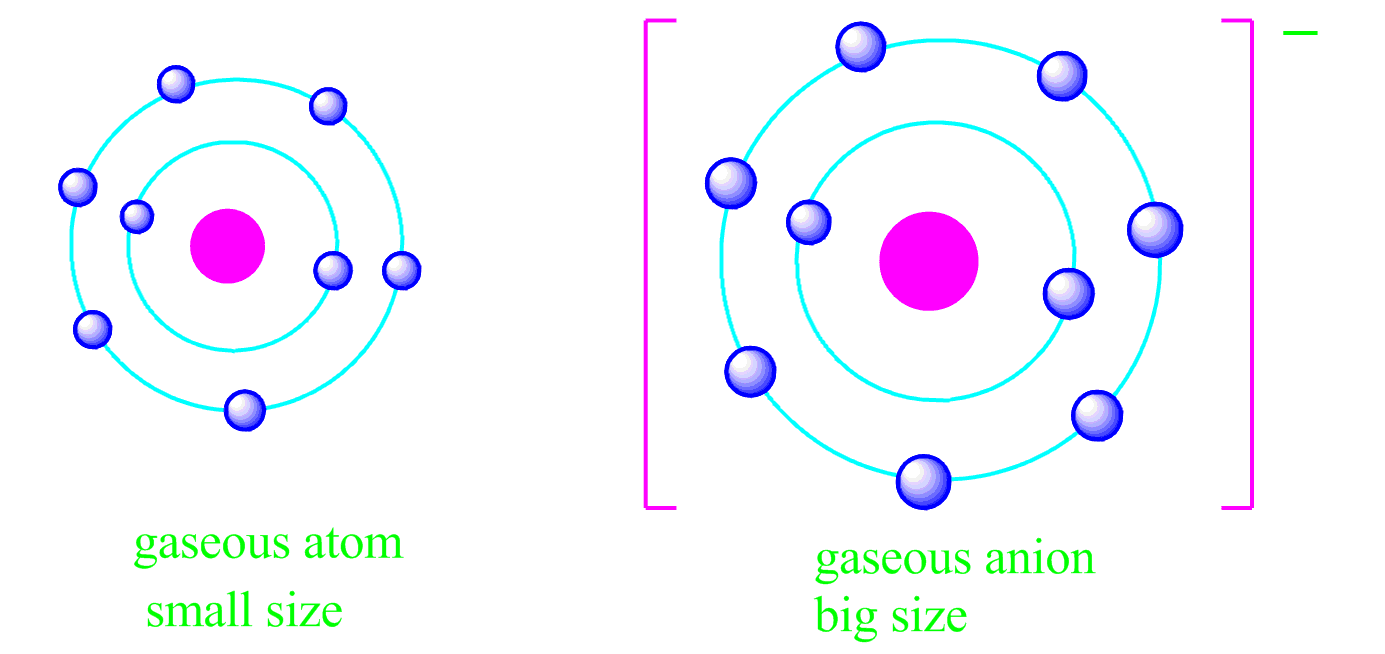
What Is Meant By Shielding Of Electrons In An Atom Class 12 . The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. Hence, the nucleus has less grip on the outer electrons and are. Effective nuclear charge (zeff) is a measure of the net positive.
from chemisfast.blogspot.com
Effective nuclear charge (zeff) is a measure of the net positive. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Hence, the nucleus has less grip on the outer electrons and are. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio.
shielding effect Slater's rules and its limitations. PG.CHEMEASY
What Is Meant By Shielding Of Electrons In An Atom Class 12 shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Hence, the nucleus has less grip on the outer electrons and are. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Effective nuclear charge (zeff) is a measure of the net positive.
From jonathanbaker.z13.web.core.windows.net
Valence Electron Configuration Chart What Is Meant By Shielding Of Electrons In An Atom Class 12 shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of.. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is Meant By Shielding Of Electrons In An Atom Class 12 The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Hence, the nucleus has less grip on the outer electrons and are. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. shielding refers to the. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Is Meant By Shielding Of Electrons In An Atom Class 12 Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Hence, the nucleus has less grip on the outer electrons and are. the shielding effect refers to the decrease in attraction between an electron and the nucleus in. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.numerade.com
SOLVEDWhat is shielding? In an atom, which electrons tend to do the What Is Meant By Shielding Of Electrons In An Atom Class 12 Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. Hence, the nucleus has less grip. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.numerade.com
SOLVED What is meant by the term "shielding of electrons" in an atom What Is Meant By Shielding Of Electrons In An Atom Class 12 Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are.. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Meant By Shielding Of Electrons In An Atom Class 12 shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of.. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 What Is Meant By Shielding Of Electrons In An Atom Class 12 The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Hence, the nucleus has less grip on the outer electrons and are. Effective nuclear charge (zeff) is a measure of the net positive. the shielding effect refers to the decrease in attraction between an electron and the nucleus in. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Meant By Shielding Of Electrons In An Atom Class 12 Hence, the nucleus has less grip on the outer electrons and are. Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. the shielding effect refers to the decrease in attraction between an electron and the nucleus in. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From answerfullnaumann.z19.web.core.windows.net
Number Of Electrons In Each Energy Level What Is Meant By Shielding Of Electrons In An Atom Class 12 Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Hence, the nucleus has less grip on the outer electrons and are. the shielding effect refers to the decrease in attraction between an electron and the nucleus in. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From mavink.com
Atomic Structure Of Matter What Is Meant By Shielding Of Electrons In An Atom Class 12 The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Effective nuclear charge (zeff) is a measure of the net positive. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. shielding refers to the core. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.scribd.com
Atoms Class XII PDF Atoms Electron What Is Meant By Shielding Of Electrons In An Atom Class 12 Hence, the nucleus has less grip on the outer electrons and are. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of.. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From slideplayer.com
Section 3 Periodic Trends ppt download What Is Meant By Shielding Of Electrons In An Atom Class 12 Hence, the nucleus has less grip on the outer electrons and are. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. Effective nuclear charge (zeff) is a measure of the net positive. shielding refers to the core electrons repelling the outer rings and thus lowering. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definition [with Examples] What Is Meant By Shielding Of Electrons In An Atom Class 12 shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. The decrease in the force of attraction exerted. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.teachoo.com
[Term 2] Account for (b) In case of transition elements, ions of same What Is Meant By Shielding Of Electrons In An Atom Class 12 Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the shielding effect refers to the decrease in attraction between an. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From surfguppy.com
What is Electronegativity? What Is Meant By Shielding Of Electrons In An Atom Class 12 shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. Effective nuclear charge (zeff) is a measure of. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Is Meant By Shielding Of Electrons In An Atom Class 12 Hence, the nucleus has less grip on the outer electrons and are. Effective nuclear charge (zeff) is a measure of the net positive. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. the shielding effect refers to the decrease in attraction between an electron and the nucleus in. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download What Is Meant By Shielding Of Electrons In An Atom Class 12 shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. the shielding effect refers to the decrease in attraction between an electron and the nucleus in any atom with more than one. Hence, the nucleus has less grip on the outer electrons and are. The decrease in the force of attraction exerted. What Is Meant By Shielding Of Electrons In An Atom Class 12.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Meant By Shielding Of Electrons In An Atom Class 12 The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of. Effective nuclear charge (zeff) is a measure of the net positive. Hence, the nucleus has less grip on the outer electrons and are. shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio.. What Is Meant By Shielding Of Electrons In An Atom Class 12.